43 structure function claims on dietary supplement labels
› health › using-dietaryUsing Dietary Supplements Wisely | NCCIH However, some types of claims related to health or the way that the product affects the structure or function of the body may appear on dietary supplement labels. Health claims describe a relationship between a substance in the supplement and reduced risk of a disease or condition. They must be based on scientific evidence. Structure Function Claims | Build Your Business, In Compliance On the other hand, certain claims, called "structure function claims", may be made about dietary supplement products and, if done correctly, will not cause these products to be subject to heightened regulation. More information about Structure Function Claims and FDA regulations may be accessed here. Thus, when marketing a FDA-regulated ...
NUTRITION SPOTLIGHT D Flashcards - Quizlet helps maintain a healthy heart Overall, the regulations around structure/function claims on dietary supplements on labels are: -informative and useful in guiding consumers to making decisions about a product's benefits. -meant to protect customers but are often misleading and overstate a product's benefits.

Structure function claims on dietary supplement labels
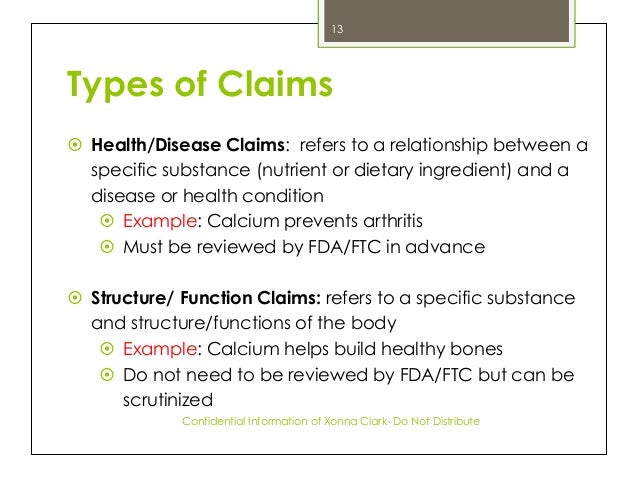
Dietary Supplement Facts Panel vs. Nutritional Facts Panel Food and supplement products can bear the following claims: health claims, nutrient content claims, and structure/function claims. While the FDA strictly regulates health claims, manufacturers have more flexibility to use nutrient and structure/function claims on labels. ... Dietary supplements can also use claims about nutrient deficiency such ... The Basis of Structure/Function Claims of Nutraceuticals Structure/function claims are labeling claims that can be used to describe the potential effects of a dietary ingredient or similar substance on the structure or function of the human body. This category of claims was created by legislation contained in the Dietary Supplement Health and Education Act. Dietary Supplements - Claims and Labeling Compliance for FDA/FTC Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds strong bones'). A structure/function claim describes the product's role in maintaining the 'structure or function of the body,' or 'general well-being.' Labeling rules are ...
Structure function claims on dietary supplement labels. PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the en.wikipedia.org › wiki › NutraceuticalNutraceutical - Wikipedia Dietary supplements, such as the vitamin B supplement shown above, are typically sold in pill form. In the United States, the Dietary Supplement Health and Education Act (DSHEA) of 1994 defined the term "dietary supplement": “A dietary supplement is a product taken by mouth that contains a 'dietary ingredient' intended to supplement the diet. How do structure/function claims differ from health claims? Subsequently, question is, what are structure and function claims? A Structure/Function Claim describe the role of a nutrient or ingredient on the structure or function of the human body. These may appear on the labels of foods, dietary supplements or drugs. Examples of a Structure/Function Claim: "Calcium builds strong bones" "Fiber maintains ... Constructing structure and function claims - Natural Products INSIDER Structure/function claims describe a nutrient's or dietary ingredient's effect on or maintenance of the structure or function of the body—for example, "calcium builds strong bones" or "antioxidants maintain cell integrity." A disease claim is an express or implied claim to diagnose, mitigate, treat, cure or prevent a disease—for ...
Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism (s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims. Solved Question 21 Structure/function claims on a dietary - Chegg question 21 structure/function claims on a dietary supplement o must acknowledge that the fda supports the claims that are misleading can lead to the product being taken off the market prove that the supplement can prevent a disease prove that the supplement can treat a disease o have to be approved, as done for drugs, before they can appear on … Federal Preemption of 'Structure/Function' Claims on Dietary Supplements With Congress finding consumers "should be empowered to make choices" about potential benefits of dietary supplements, DSHEA implemented major shifts in dietary supplement regulation, including exempting "dietary supplements" from FDA drug approval and FDA food additive approval, 21 U.S.C. §321(g)(1), and expressly permitting dietary ... Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims en.wikipedia.org › wiki › Dietary_Supplement_HealthDietary Supplement Health and Education Act of 1994 DSHEA and other federal regulations require the following information to appear on dietary supplement labels: a statement of identity that contains the words "dietary supplement." The word "dietary" may be replaced by the name of the dietary ingredient (e.g., "ginseng supplement") net quantity of contents (for example, "60 capsules") FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in... › structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ...
FDA Issues Final Rules for Structure/Function Claims for Dietary ... On January 6, 2000, the Food and Drug Administration (FDA) issued its final regulations on structure/function (SF) claims for dietary supplements (DS) under the Dietary Supplement Health and Education Act of 1994 (DSHEA). (1) The full text is available on the Internet. (2)
Structure/Function Claims in Dietary Supplement Labeling: Not All of ... One important means of promoting dietary supplements is the inclusion, in label ing, of claims about the impact on the structure or function of the human body. These claims are known as "structure/function" claims. The author has observed the evolu tion of the Food and Drug Administration's (FDA's) regulation of dietary supplement
Structure Function Claims: study guides and answers on Quizlet Our most recent study sets focusing on Structure Function Claims will help you get ahead by allowing you to study whenever you want, wherever you are. Exam 1- Ch. 1-4, 18 336 terms tanyatovar Nutrition chapter 2 31 terms michael_pang4 Chap 2- Nutrition 23 terms esssssmeralda Chapter 2 21 terms dalabadi NUTITION 251 FINAL EXAM VOCAB 86 terms
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, …
Structure/Function Claim Notification for Dietary Supplements ... The law states that a dietary supplement may bear certain statements on its label or in its labeling if the claim (s) meets certain requirements, if the entities making the claims have...
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels...
Dietary Supplements: An Advertising Guide for Industry Application of FTC Law to Dietary Supplement Advertising A. Identifying Claims and Interpreting Ad Meaning 1. Identifying Express and Implied Claims 2. When to Disclose Qualifying Information 3. Clear and Prominent Disclosure B. Substantiating Claims 1. Overview 2. Ads that Refer to a Specific Level of Support 3. The Amount and Type of Evidence 4.
''Structure/Function'' Claims in Dietary Supplement Labeling Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function' claims on...




Post a Comment for "43 structure function claims on dietary supplement labels"