45 fda guidance use of symbols on labels
Guidance for the Use of Bayesian Statistics in Medical Device Clinical Feb 05, 2010 · This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or ... Dosage Form Drug Manufacturers cGMPs (10/93) | FDA Use of gang-printed labels will be prohibited unless they are adequately differentiated by size, shape or color. (211.122(f)) If cut labels are used one of the following special control procedures ...
Medication Errors Related to CDER-Regulated Drug Products | FDA Sep 08, 2021 · FDA draft guidance for industry Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors. April 2013. April 2013. When final, this guidance will ...

Fda guidance use of symbols on labels
› food › food-labeling-nutritionUse of the Term Healthy on Food Labeling | FDA Mar 25, 2022 · The FDA has started a public process to update the "healthy" nutrient content claim for food labeling. Updating "healthy" is part of an overall plan to provide consumers with information and tools ... › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ... Fda Announces Guidance for Symbol Use on Ivd Labels The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices.
Fda guidance use of symbols on labels. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Mar 29, 2022 · The words intended uses or words of similar import in §§ 801.5, 801.119, 801.122, and 1100.5 of this chapter refer to the objective intent of the persons legally responsible for the labeling of an article (or their representatives). The intent may be shown by such persons' expressions, the design or composition of the article, or by the circumstances surrounding the … Use of Symbols in Labeling | FDA Use of Symbols in Labeling The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits... Summary: Use of Symbols in Labeling (Final Rule) | FDA The Food and Drug Administration (FDA or the Agency) is issuing this final rule revising its medical device and certain biological product labeling regulations to explicitly allow for the optional ... EOF
Use of the Term Healthy on Food Labeling | FDA Mar 25, 2022 · The FDA has started a public process to update the "healthy" nutrient content claim for food labeling. Updating "healthy" is part of an overall plan to provide consumers with information and tools ... › about-fda › economic-impact-analysesSummary: Use of Symbols in Labeling (Final Rule) | FDA The Food and Drug Administration (FDA or the Agency) is issuing this final rule revising its medical device and certain biological product labeling regulations to explicitly allow for the optional ... › inspections-compliance-enforcementDosage Form Drug Manufacturers cGMPs (10/93) | FDA Use of gang-printed labels will be prohibited unless they are adequately differentiated by size, shape or color. (211.122(f)) If cut labels are used one of the following special control procedures ... Use of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14.
Small Entity Compliance Guide on Structure/Function Claims | FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ... FDA Final Rule on the Use of Symbols in Labeling - loring In an attempt to remedy this issue and harmonize the U.S. device labeling requirements with international regulatory requirements (e.g., ISO 15223-1:2021 and BS EN 980:2008), the FDA has issued a final rule that revises its "medical device (and certain biological product) labeling regulations to explicitly allow for the optional inclusion of graphical representations of information, or ... FDA Issues Final Rule Permitting Use of Symbols on Device Labeling Three years ago, FDA proposed a rule that would permit device manufacturers to use stand‑alone symbols on device labeling. In the meantime, this issue has continued to fester. So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways: › medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA The Use of Symbols in Medical Device Labeling Final Rule ("final rule"), including the requirement for a glossary, only applies to symbols that are used to convey information required by or under...
Federal Register :: Use of Symbols in Labeling accordingly, this final rule provides that a stand-alone symbol is allowed to be used in device labeling if: (1) the symbol is established in a standard developed by an sdo; and (2) the standard is recognized by fda under its authority under section 514 (c) of the fdc act and the symbol is used according to the specifications for use of the …

Statement from ASH Executive Director Laurent Huber on FDA and Graphic Warning Labels: Glacial ...
Food/Dietary Supplement Guidance and Regulatory Information May 16, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ...
Using Symbols to Convey Information in Medical Device Labeling In June, FDA issued the Use of Symbols in Labeling final rule, which describes the circumstances in which manufacturers can use a stand-alone symbol in device labeling without any adjacent explanatory text. For example, if certain requirements are met under the final rule, manufacturers of sterile syringes could opt to use the symbol for "do ...
› regulatory-information › search-fdaGuidance for the Use of Bayesian Statistics in Medical Device ... Feb 05, 2010 · This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or ...
Withdrawn Guidance | FDA - U.S. Food and Drug Administration Jul 07, 2022 · Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use: 11/30/2004: ... Guidance for FDA Staff: Civil Money Penalty Policy: 06/08/1999:
Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by FDA's labeling requirements for IVDs. This draft guidance is not final nor is it in effect at this time. DATES:
› food › guidance-regulation-food-andFood/Dietary Supplement Guidance and Regulatory Information May 16, 2022 · WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process ...
FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS - FDAnews FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS December 2, 2004 The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices. To View This Article: Login
PDF Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Device ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553.
Fda Announces Guidance for Symbol Use on Ivd Labels The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices.
› regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ...
› food › food-labeling-nutritionUse of the Term Healthy on Food Labeling | FDA Mar 25, 2022 · The FDA has started a public process to update the "healthy" nutrient content claim for food labeling. Updating "healthy" is part of an overall plan to provide consumers with information and tools ...






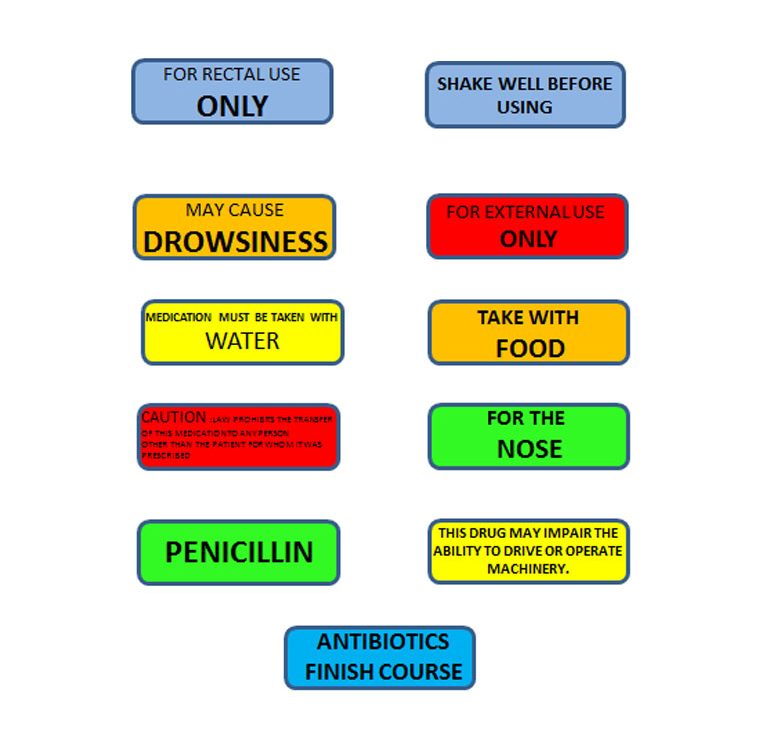
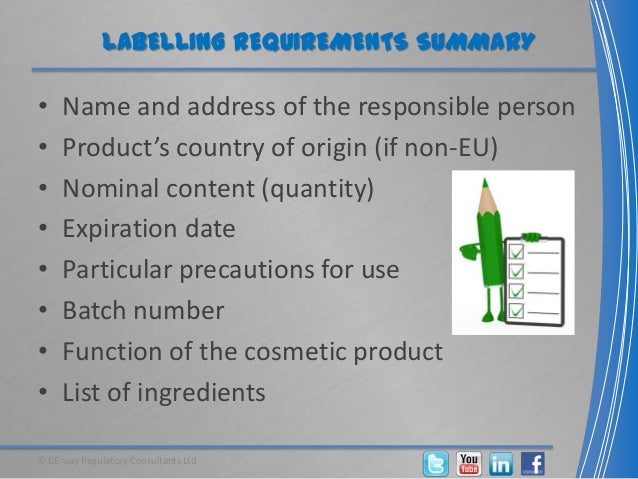
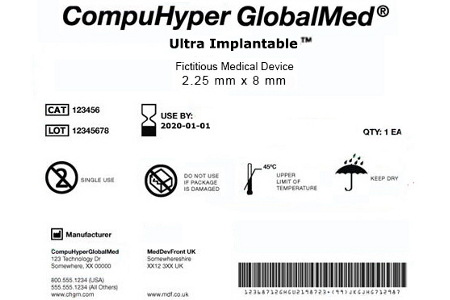


Post a Comment for "45 fda guidance use of symbols on labels"